How is Avogadro's law used in everyday life?
Avogadro, in 1811, first gave a hypothesis which subsequently was turned into a law. He presumed that the elementary gases like nitrogen, hydrogen, oxygen etc. Exist as diatomic molecules. His presumption was later experimentally confirmed by Cannizzaro in 1950 after which the hypothesis turned into a law. There are four laws, known as Gas Laws, which describe how gases behave.The four laws are Boyle’s Law, Charles’s Law, Gay-Lussac’s Law and Avogadro’s Law. Avogadro’s Law Amadeo Avogadro was an Italian physicist who stated, in 1811, that the volume of any gas is proportional to the number of molecules of gas (measured in Moles – symbol mol).
1 Answer

Avogadro's Law Ppt
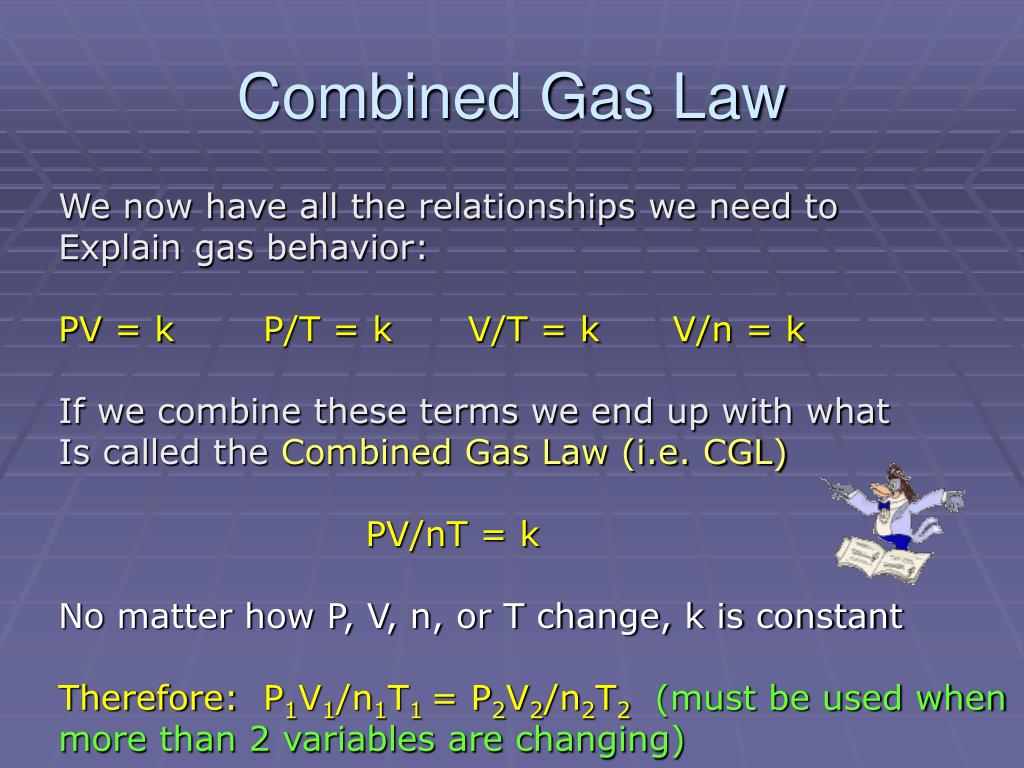
Avogadro's Law states that the volume of a gas is directly proportional to the number of moles of gas.
Here are some examples.
Avogadro's Law Powerpoint
As you blow up a basketball, you are forcing more gas molecules into it. The more molecules, the greater the volume. The basketball inflates.
A flat tire takes up less space than an inflated tire, because it contains less air.
Lungs expand as they fill with air. Exhaling decreases the volume of the lungs.

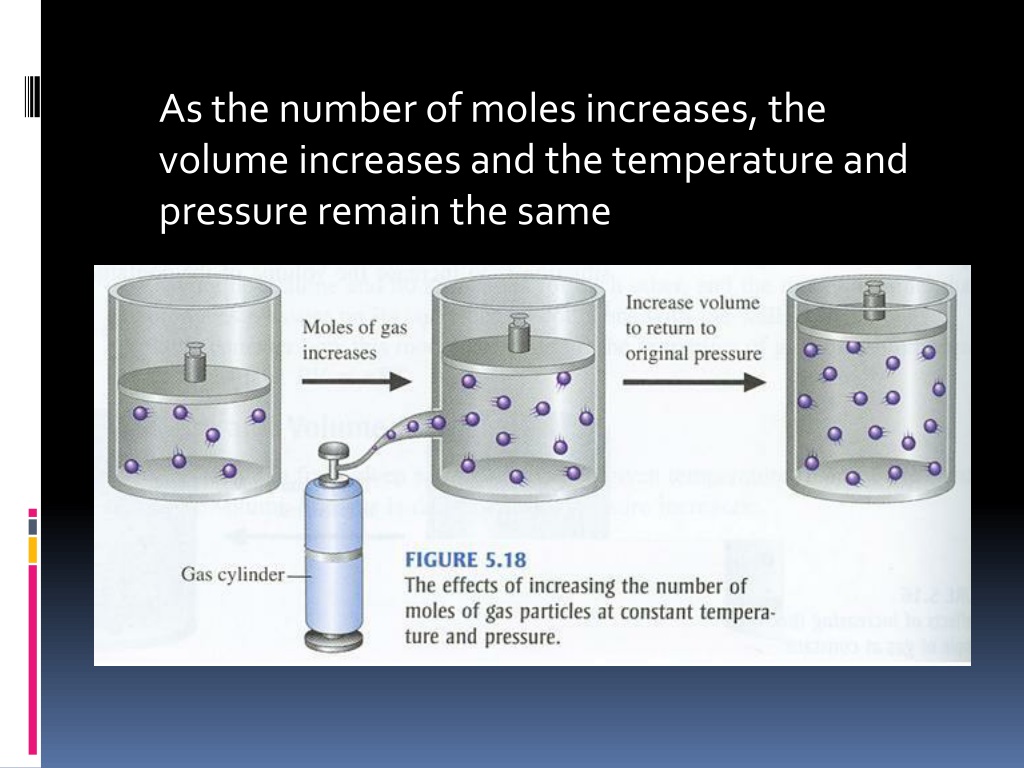
Avogadro's Law Real Life Example
A balloon filled with helium weighs much less than an identical balloon filled with air. Both balloons contain the same number of molecules. Helium atoms have lower mass than either oxygen molecules or nitrogen molecules in air, so the helium balloon is lighter.
Avogadro's Law Calculator
Hope this helps.
Avogadro's Gas Law Ppt
Related questions

